Fast-Track Your Medical Device Approval with Comprehensive Biocompatibility Testing
ISO 17025 Accredited & GLP Certified Labs. We handle the entire testing process for MDR and FDA compliance – delivering results on time, with zero hidden costs.

From A to Z Chemical Characterization
& Biocompatibility
From BEP (Biological Evaluation Plan) to final BER (Biological Evaluation Report), we manage the whole testing process under ISO 10993 standards.
Comprehensive Testing Scope (All-inclusive ISO 10993 testing services)
Chemical Characterization
Toxicological Risk Assessment
Consulting & Strategy (Avoid unnecessary tests)
Global Reach, Local Touch: Operating as EBI in Europe and NABI in the USA, we combine 20+ years of expertise with a state-of-the-art laboratory to reduce your costs and delivery times.
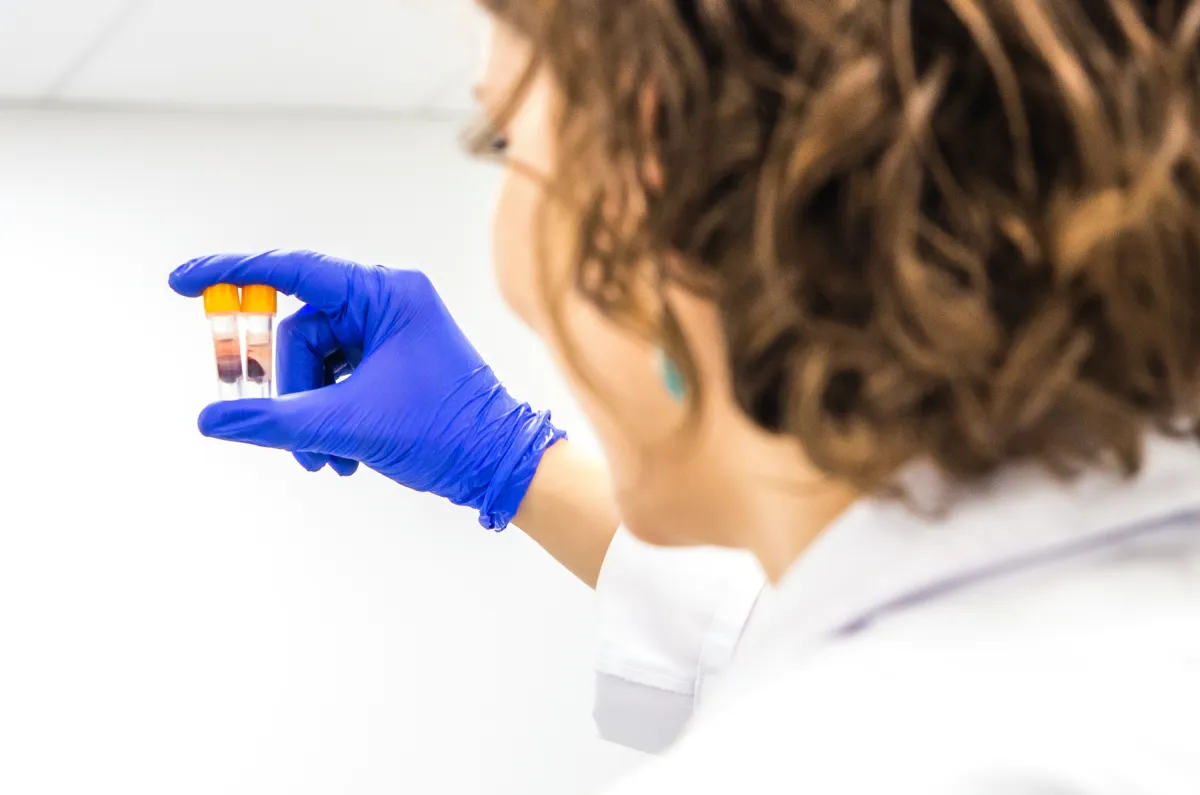
Tired of Waiting for Results?
- Speed Matters - Over 99% of our reports are delivered before the deadline. No massive backlogs. We start your tests immediately after sample reception.
- Global Acceptance - Our reports are recognized by Competent Authorities and Notified Bodies worldwide. Whether you target CE marking or FDA approval, we’ve got you covered.
- Free Regulatory Support - We don't just hand you a report and leave. We support you with Notified Body queries on testing free of charge until your registration is complete.
We know that in the medical device industry, time is money! While other labs struggle with backlogs and slow communication, EBI & NABI deliver certainty.

Your Path to Compliance in 4 Simple Phases
We are specialized in comprehensive biocompatibility testing according to the all series of ISO 10993 biological evaluation of medical devices – but not only! We are constantly developing our labs to offer more services for your medical devices:
- Microbiology and sterility testing
- Package validation
- Reprocessing validation
- Consulting services
Why Us?
European Biomedical Institute profile
We always pay attention to our Customers needs. From the beginning of our history till today, our aim is to offer the highest quality of tests with the shortest possible lead time in a cost effective manner. This is our DNA.
Clients story

Contact with us
We’re open to collaboration and research partnerships. Fill out the form — our team will respond as soon as possible.
Get in touch

